Page 147 - DJJ20063- Thermodynamics 1
P. 147
DJJ20063- Thermodynamics 1
Example 4.2
What is the highest possible theoretical efficiency of a Carnot cycle with a hot
o
reservoir of steam at 200 C when the cooling water available from condenser is at
o
10 C?
Solution to Example 4.2
From equation 4.8,
T (s ) s −
carnot = 1− 2 B A ) s −
(s T
1 B A
10 + 273
= 1−
200 + 273
283
= 1−
473
i.e. Highest possible efficiency = 1 – 0.598
= 0.402 or 40.2 %
Example 4.3
A steam power plant operates between a boiler pressure of 42 bar and a condenser
pressure of 0.035 bar. Calculate for these limits the cycle efficiency and the work
ratio for a Carnot cycle using wet steam.
Solution to Example 4.3
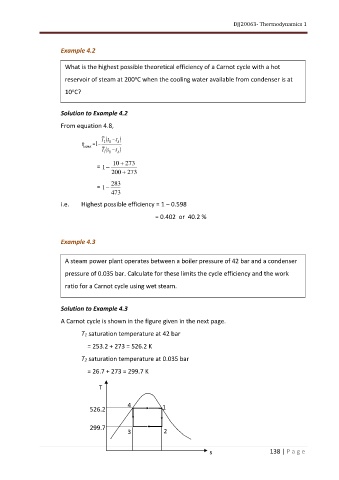
A Carnot cycle is shown in the figure given in the next page.
T1 saturation temperature at 42 bar
= 253.2 + 273 = 526.2 K
T2 saturation temperature at 0.035 bar
= 26.7 + 273 = 299.7 K
T
4
526.2 1
299.7 2
3
s 138 | P a g e