Page 77 - DJJ20063- Thermodynamics 1
P. 77
DJJ20063- Thermodynamics 1
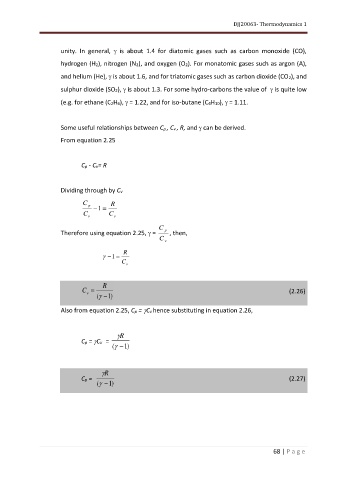
unity. In general, is about 1.4 for diatomic gases such as carbon monoxide (CO),
hydrogen (H2), nitrogen (N2), and oxygen (O2). For monatomic gases such as argon (A),
and helium (He), is about 1.6, and for triatomic gases such as carbon dioxide (CO2), and
sulphur dioxide (SO2), is about 1.3. For some hydro-carbons the value of is quite low
(e.g. for ethane (C2H6), = 1.22, and for iso-butane (C4H10), = 1.11.
Some useful relationships between Cp , Cv , R, and can be derived.
From equation 2.25
Cp - Cv= R
Dividing through by Cv
C R
p −1 =
C v C v
C
Therefore using equation 2.25, = p , then,
C v
R
−1 =
C
v
R
C v = (2.26)
( − ) 1
Also from equation 2.25, Cp = Cv hence substituting in equation 2.26,
R
Cp = Cv =
( − ) 1
R
Cp = (2.27)
( − ) 1
68 | P a g e