Page 118 - DJJ20063- Thermodynamics 1
P. 118
DJJ20063- Thermodynamics 1
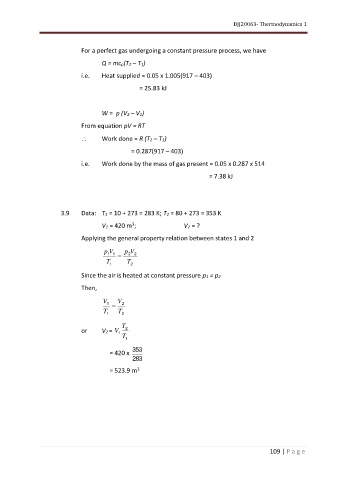
For a perfect gas undergoing a constant pressure process, we have
Q = mcp(T2 – T1)
i.e. Heat supplied = 0.05 x 1.005(917 – 403)
= 25.83 kJ
W = p (V2 – V1)
From equation pV = RT
Work done = R (T2 – T1)
= 0.287(917 – 403)
i.e. Work done by the mass of gas present = 0.05 x 0.287 x 514
= 7.38 kJ
3.9 Data: T1 = 10 + 273 = 283 K; T2 = 80 + 273 = 353 K
3
V1 = 420 m ; V2 = ?
Applying the general property relation between states 1 and 2
p V p V
1 1 = 2 2
T 1 T 2
Since the air is heated at constant pressure p1 = p2
Then,
V V
1 = 2
T 1 T 2
T
or V2 = V 1 2
T 1
353
= 420 x
283
3
= 523.9 m
109 | P a g e