Page 24 - DJJ20063- Thermodynamics 1
P. 24
DJJ20063- Thermodynamics 1
Liquid Phase
In the liquid phase, the molecules are;
(a) closely bound, therefore also relatively dense and unable to expand to fill a
space; but
(b) they are no longer rigidly structured so much so that they are free to move
within a fixed volume. An example is a pure liquid state.
Steam Phase
In the steam phase, the molecules;
(a) virtually do not attract each other. The distance between the molecules are not
as close as those in the solid and liquid phases;
(b) are not arranged in a fixed pattern. There is neither a fixed volume nor a fixed
shape for steam.
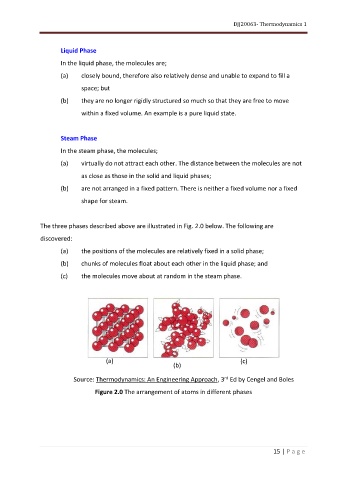
The three phases described above are illustrated in Fig. 2.0 below. The following are
discovered:
(a) the positions of the molecules are relatively fixed in a solid phase;
(b) chunks of molecules float about each other in the liquid phase; and
(c) the molecules move about at random in the steam phase.
(a) (c)
(b)
rd
Source: Thermodynamics: An Engineering Approach, 3 Ed by Cengel and Boles
Figure 2.0 The arrangement of atoms in different phases
15 | P a g e