Page 29 - DJJ20063- Thermodynamics 1
P. 29
DJJ20063- Thermodynamics 1
2.2.3 Draw, illustrate and label the property diagram for phase- Changes
process; saturated vapour, superheated vapour, quality of vapour.
o
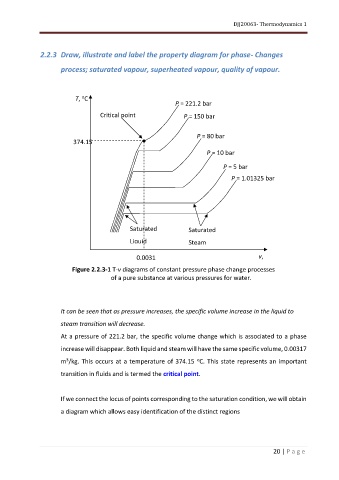
T, C
P = 221.2 bar
Critical point P = 150 bar
P = 80 bar
374.15
P = 10 bar
P = 5 bar
P = 1.01325 bar
Saturated Saturated
Liquid Steam
0.0031 v,
3
m /kg
Figure 2.2.3-1 T-v diagrams of constant pressure phase change processes
7
of a pure substance at various pressures for water.
It can be seen that as pressure increases, the specific volume increase in the liquid to
steam transition will decrease.
At a pressure of 221.2 bar, the specific volume change which is associated to a phase
increase will disappear. Both liquid and steam will have the same specific volume, 0.00317
3
o
m /kg. This occurs at a temperature of 374.15 C. This state represents an important
transition in fluids and is termed the critical point.
If we connect the locus of points corresponding to the saturation condition, we will obtain
a diagram which allows easy identification of the distinct regions
20 | P a g e