Page 31 - DJJ20063- Thermodynamics 1
P. 31
DJJ20063- Thermodynamics 1
Activity 2A
TEST YOUR UNDERSTANDING BEFORE YOU CONTINUE WITH THE NEXT INPUT…!
2.1 Each line in the table below gives information about phases of pure substances. Fill in
the phase column in the table with the correct answer.
Statement Phase
The molecules are closely bound, they are also relatively dense and
unable to expand to fill a space. However they are no longer rigidly i._________
structured so that they are free to move within a fixed volume.
The molecules are closely bound, they are relatively dense and
arranged in a rigid three-dimensional patterns so that they do not ii.________
easily deform.
The molecules virtually do not attract each other. The distance
between the molecules are not as close as those in the solid and
liquid phases. They are not arranged in a fixed pattern. There is iii.________
neither a fixed volume nor a fixed shape for steam.
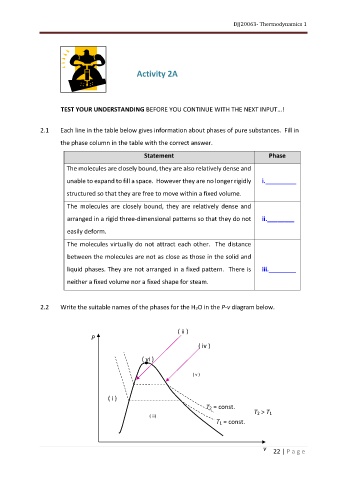
2.2 Write the suitable names of the phases for the H2O in the P-v diagram below.
( ii )
P
( iv )
( vi )
( v )
( i )
T2 = const.
T2 > T1
( iii)
T1 = const.
v
22 | P a g e