Page 26 - DJJ20063- Thermodynamics 1
P. 26
DJJ20063- Thermodynamics 1
STATE 1 STATE 2 STATE 3 STATE 4
W
W
W
W
Liquid Steam Superheate
d
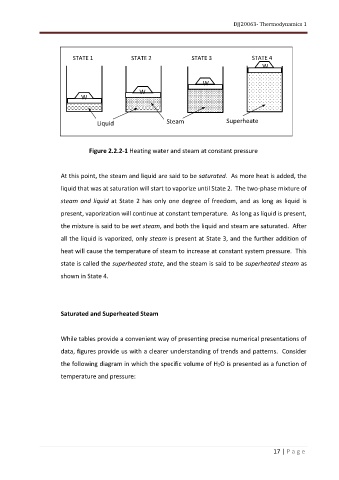
Figure 2.2.2-1 Heating water and steam at constant pressure
At this point, the steam and liquid are said to be saturated. As more heat is added, the
liquid that was at saturation will start to vaporize until State 2. The two-phase mixture of
steam and liquid at State 2 has only one degree of freedom, and as long as liquid is
present, vaporization will continue at constant temperature. As long as liquid is present,
the mixture is said to be wet steam, and both the liquid and steam are saturated. After
all the liquid is vaporized, only steam is present at State 3, and the further addition of
heat will cause the temperature of steam to increase at constant system pressure. This
state is called the superheated state, and the steam is said to be superheated steam as
shown in State 4.
Saturated and Superheated Steam
While tables provide a convenient way of presenting precise numerical presentations of
data, figures provide us with a clearer understanding of trends and patterns. Consider
the following diagram in which the specific volume of H2O is presented as a function of
temperature and pressure:
17 | P a g e