Page 66 - DJJ20063- Thermodynamics 1
P. 66
DJJ20063- Thermodynamics 1
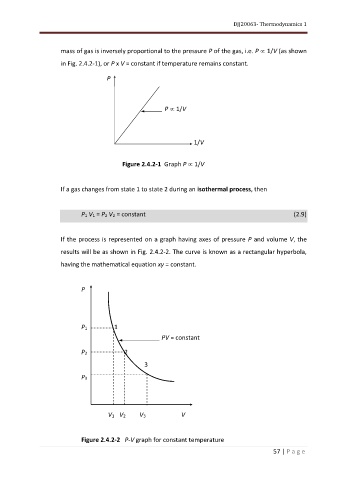
mass of gas is inversely proportional to the pressure P of the gas, i.e. P 1/V (as shown
in Fig. 2.4.2-1), or P x V = constant if temperature remains constant.
P
P 1/V
1/V
Figure 2.4.2-1 Graph P 1/V
If a gas changes from state 1 to state 2 during an isothermal process, then
P1 V1 = P2 V2 = constant (2.9)
If the process is represented on a graph having axes of pressure P and volume V, the
results will be as shown in Fig. 2.4.2-2. The curve is known as a rectangular hyperbola,
having the mathematical equation xy = constant.
P
P1 1
PV = constant
P2 2
3
P3
V1 V2 V3 V
Figure 2.4.2-2 P-V graph for constant temperature
57 | P a g e