Page 63 - DJJ20063- Thermodynamics 1
P. 63
DJJ20063- Thermodynamics 1
2.3.5 Determine the heat, work, and internal energy for the above process.
To show that Q = h2 – h1
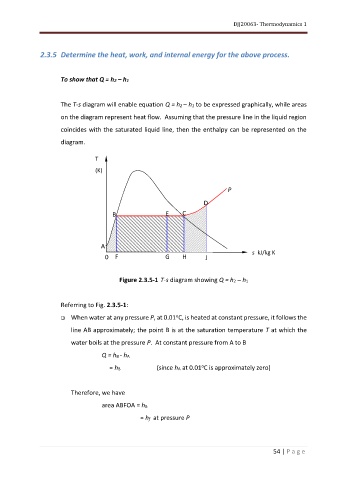
The T-s diagram will enable equation Q = h2 – h1 to be expressed graphically, while areas
on the diagram represent heat flow. Assuming that the pressure line in the liquid region
coincides with the saturated liquid line, then the enthalpy can be represented on the
diagram.
T
(K)
P
D
B E C
A
0 F G H J s kJ/kg K
Figure 2.3.5-1 T-s diagram showing Q = h2 – h1
Referring to Fig. 2.3.5-1:
o
❑ When water at any pressure P, at 0.01 C, is heated at constant pressure, it follows the
line AB approximately; the point B is at the saturation temperature T at which the
water boils at the pressure P. At constant pressure from A to B
Q = hB - hA
o
= hB (since hA at 0.01 C is approximately zero)
Therefore, we have
area ABFOA = hB
= hf at pressure P
54 | P a g e