Page 62 - DJJ20063- Thermodynamics 1
P. 62
DJJ20063- Thermodynamics 1
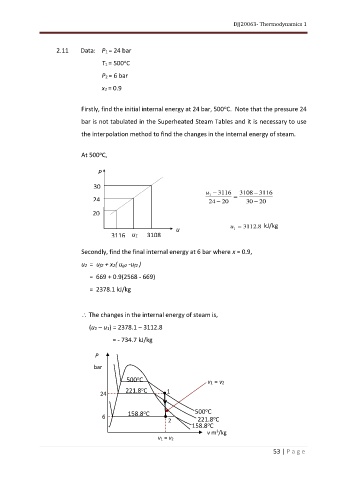
2.11 Data: P1 = 24 bar
o
T1 = 500 C
P2 = 6 bar
x2 = 0.9
o
Firstly, find the initial internal energy at 24 bar, 500 C. Note that the pressure 24
bar is not tabulated in the Superheated Steam Tables and it is necessary to use
the interpolation method to find the changes in the internal energy of steam.
o
At 500 C,
P
30
u − 3116 3108 − 3116
1 =
24 24 − 20 30 − 20
20
u = 3112 8 . kJ/kg
u 1
3116 u1 3108
Secondly, find the final internal energy at 6 bar where x = 0.9,
u2 = uf2 + x2( ug2 -uf2 )
= 669 + 0.9(2568 - 669)
= 2378.1 kJ/kg
The changes in the internal energy of steam is,
(u2 – u1) = 2378.1 – 3112.8
= - 734.7 kJ/kg
P
bar
500 C
o
v1 = v2
o
221.8 C 1
24
o
o
6 158.8 C 500 C o
2 221.8 C
o
158.8 C
3
v m /kg
v1 = v2
53 | P a g e