Page 98 - DJJ20063- Thermodynamics 1
P. 98
DJJ20063- Thermodynamics 1
3.2 Data: m=1kg; M= 28 kg/kmole p1 = 1.01 bar;
T1 = 20 + 272 = 293 K; p2 = 4.2 bar
From equation
R
R = 0
M
. 8 314
=
28
= 0.297 kJ/kgK
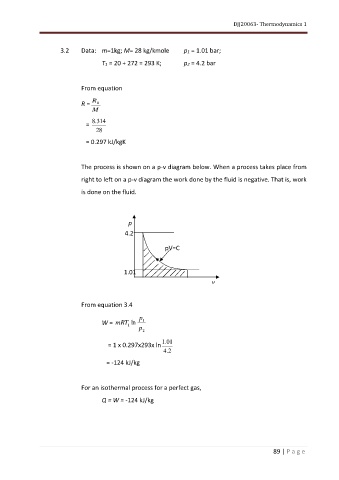
The process is shown on a p-v diagram below. When a process takes place from
right to left on a p-v diagram the work done by the fluid is negative. That is, work
is done on the fluid.
p
4.2
pV=C
1.01
v
From equation 3.4
p
W = mRT 1 ln 1
p 2
. 1 01
= 1 x 0.297x293x ln
2 . 4
= -124 kJ/kg
For an isothermal process for a perfect gas,
Q = W = -124 kJ/kg
89 | P a g e