Page 109 - DJJ20063- Thermodynamics 1
P. 109
DJJ20063- Thermodynamics 1
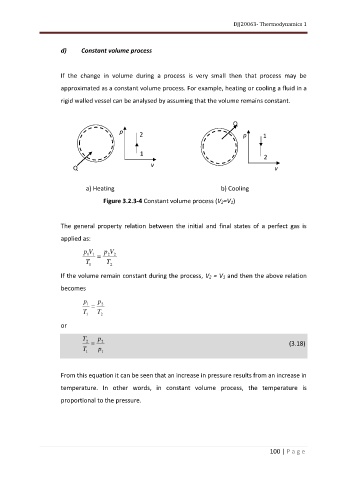
d) Constant volume process
If the change in volume during a process is very small then that process may be
approximated as a constant volume process. For example, heating or cooling a fluid in a
rigid walled vessel can be analysed by assuming that the volume remains constant.
Q
p 2
p 1
1
2
v
Q v
a) Heating b) Cooling
Figure 3.2.3-4 Constant volume process (V2=V1)
The general property relation between the initial and final states of a perfect gas is
applied as:
p V p V
1 1 = 2 2
T 1 T 2
If the volume remain constant during the process, V2 = V1 and then the above relation
becomes
p p
1 = 2
T 1 T 2
or
T p
2 = 2 (3.18)
T 1 p 1
From this equation it can be seen that an increase in pressure results from an increase in
temperature. In other words, in constant volume process, the temperature is
proportional to the pressure.
100 | P a g e