Page 110 - DJJ20063- Thermodynamics 1
P. 110
DJJ20063- Thermodynamics 1
Work transfer:
Work transfer (pdV) must be zero because the change in volume, dV, during the process
is zero. However, work in the form of paddle-wheel work may be transferred.
Heat transfer:
Applying the non flow energy equation
Q – W = U2 – U1
gives Q – 0 = U2 – U1
i.e. Q = U2 – U1 (3.19)
This result, which is important and should be remembered, shows that the nett amount
of heat energy supplied to or taken from a fluid during a constant volume process is equal
to the change in the internal energy of the fluid.
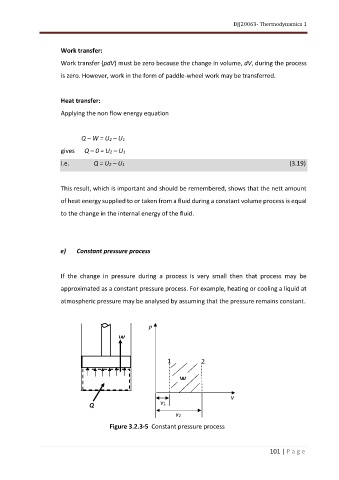
e) Constant pressure process
If the change in pressure during a process is very small then that process may be
approximated as a constant pressure process. For example, heating or cooling a liquid at
atmospheric pressure may be analysed by assuming that the pressure remains constant.
P
W
1 2
W
v
Q v1
v2
Figure 3.2.3-5 Constant pressure process
101 | P a g e