Page 48 - DJJ20063- Thermodynamics 1
P. 48
DJJ20063- Thermodynamics 1
Example 2.5
3
Steam at 100 bar has a specific volume of 0.02812 m /kg. Find the
temperature, degree of superheat, specific enthalpy and specific internal
energy.
Solution to Example 2.5
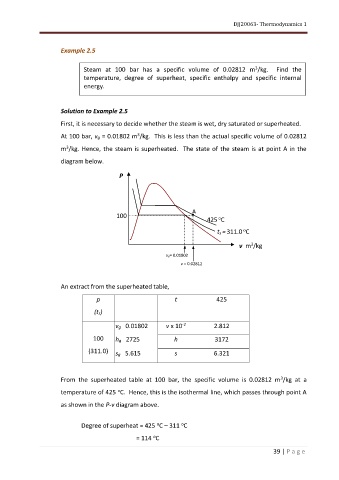
First, it is necessary to decide whether the steam is wet, dry saturated or superheated.
3
At 100 bar, vg = 0.01802 m /kg. This is less than the actual specific volume of 0.02812
3
m /kg. Hence, the steam is superheated. The state of the steam is at point A in the
diagram below.
P
ba
A
100 o
425 C
o
ts = 311.0 C
3
v m /kg
vg= 0.01802
v = 0.02812
An extract from the superheated table,
p t 425
(ts)
vg 0.01802 v x 10 -2 2.812
100 hg 2725 h 3172
(311.0) sg 5.615 s 6.321
3
From the superheated table at 100 bar, the specific volume is 0.02812 m /kg at a
o
temperature of 425 C. Hence, this is the isothermal line, which passes through point A
as shown in the P-v diagram above.
o
o
Degree of superheat = 425 C – 311 C
o
= 114 C
39 | P a g e