Page 90 - DJJ20063- Thermodynamics 1
P. 90
DJJ20063- Thermodynamics 1
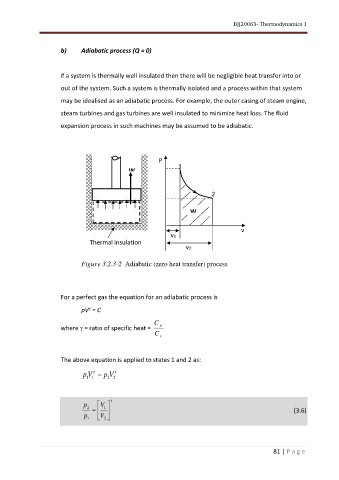
b) Adiabatic process (Q = 0)
If a system is thermally well insulated then there will be negligible heat transfer into or
out of the system. Such a system is thermally isolated and a process within that system
may be idealised as an adiabatic process. For example, the outer casing of steam engine,
steam turbines and gas turbines are well insulated to minimize heat loss. The fluid
expansion process in such machines may be assumed to be adiabatic.
P
W 1
2
W
v1 v
Thermal insulation
v2
Figure 3.2.3-2 Adiabatic (zero heat transfer) process
For a perfect gas the equation for an adiabatic process is
pV = C
C
where = ratio of specific heat = p
C v
The above equation is applied to states 1 and 2 as:
p 1 V = p 2 V
1
2
p V
2 = 1 (3.6)
V
p 1 2
81 | P a g e