Page 87 - DJJ20063- Thermodynamics 1
P. 87
DJJ20063- Thermodynamics 1
P
1
W
2
W
v
v1
Q
v2
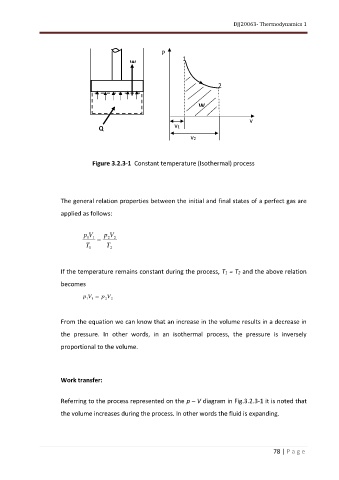
Figure 3.2.3-1 Constant temperature (Isothermal) process
The general relation properties between the initial and final states of a perfect gas are
applied as follows:
p V p V
1 1 = 2 2
T 1 T 2
If the temperature remains constant during the process, T1 = T2 and the above relation
becomes
p 1 V = p 2 V
1
2
From the equation we can know that an increase in the volume results in a decrease in
the pressure. In other words, in an isothermal process, the pressure is inversely
proportional to the volume.
Work transfer:
Referring to the process represented on the p – V diagram in Fig.3.2.3-1 it is noted that
the volume increases during the process. In other words the fluid is expanding.
78 | P a g e