Page 84 - DJJ20063- Thermodynamics 1
P. 84
DJJ20063- Thermodynamics 1
3.1.2 Describe energy transfer by heat and energy transfer by work.
a ) Work
Work transfer is defined as a product of the force and the distance moved in the
direction of the force. When a boundary of a close system moves in the direction
of the force acting on it, then the system does work on its surroundings. When the
boundary is moved inwards the work is done on the system by its surroundings.
The units of work are, for example, Nm or J. If work is done on unit mass of a
fluid, then the work done per kg of fluid has the units of Nm/kg or J/kg. Consider
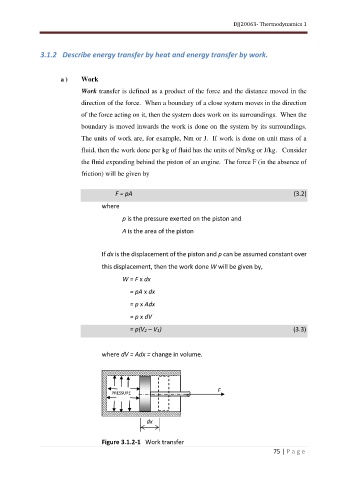
the fluid expanding behind the piston of an engine. The force F (in the absence of
friction) will be given by
F = pA (3.2)
where
p is the pressure exerted on the piston and
A is the area of the piston
If dx is the displacement of the piston and p can be assumed constant over
this displacement, then the work done W will be given by,
W = F x dx
= pA x dx
= p x Adx
= p x dV
= p(V2 – V1) (3.3)
where dV = Adx = change in volume.
S
PRESSURE F
I
S
T dx
E
Figure 3.1.2-1 Work transfer
M
75 | P a g e