Page 85 - DJJ20063- Thermodynamics 1
P. 85
DJJ20063- Thermodynamics 1
When two systems at different temperatures are in contact with each other,
energy will transfer between them until they reach the same temperature (that
is, when they are in equilibrium with each other). This energy is called heat, or
thermal energy, and the term "heat flow" refers to an energy transfer as a
consequence of a temperature difference.
b ) Heat
Heat is a form of energy which crosses the boundary of a system during a change
of state produced by the difference in temperature between the system and its
surroundings. The unit of heat is taken as the amount of heat energy equivalent
to one joule or Nm. The joule is defined as the work done when the point of
application of a force of one newton is displaced through a distance of one meter
in the direction of the force.
3.2 Apply the concept of the first law of thermodynamics.
3.2.1 Define of a closed system.
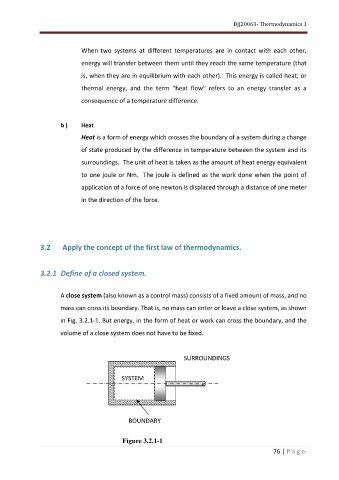
A close system (also known as a control mass) consists of a fixed amount of mass, and no
mass can cross its boundary. That is, no mass can enter or leave a close system, as shown
in Fig. 3.2.1-1. But energy, in the form of heat or work can cross the boundary, and the
volume of a close system does not have to be fixed.
SURROUNDINGS
SYSTEM
BOUNDARY
Figure 3.2.1-1
76 | P a g e