Page 56 - DJJ20063- Thermodynamics 1
P. 56
DJJ20063- Thermodynamics 1
o
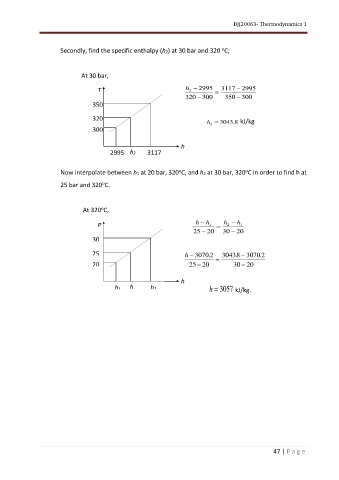
Secondly, find the specific enthalpy (h2) at 30 bar and 320 C;
At 30 bar,
T h 2 − 2995 = 3117 − 2995
320 − 300 350 − 300
350
320
h = 3043 8 . kJ/kg
2
300
h
2995 h2 3117
o
o
Now interpolate between h1 at 20 bar, 320 C, and h2 at 30 bar, 320 C in order to find h at
o
25 bar and 320 C.
o
At 320 C,
h − h h − h
P 1 = 2 1
25 − 20 30 − 20
30
25 h − 3070 2 . 30438 . − 3070 2 .
20 25 − 20 = 30 − 20
h
h1 h h2 h = 3057 kJ/kg.
47 | P a g e